PROSTOX is Clinically Validated to Help Physicians Identify Patients at Risk of Developing Significant Late Genitourinary (GU) Toxicity
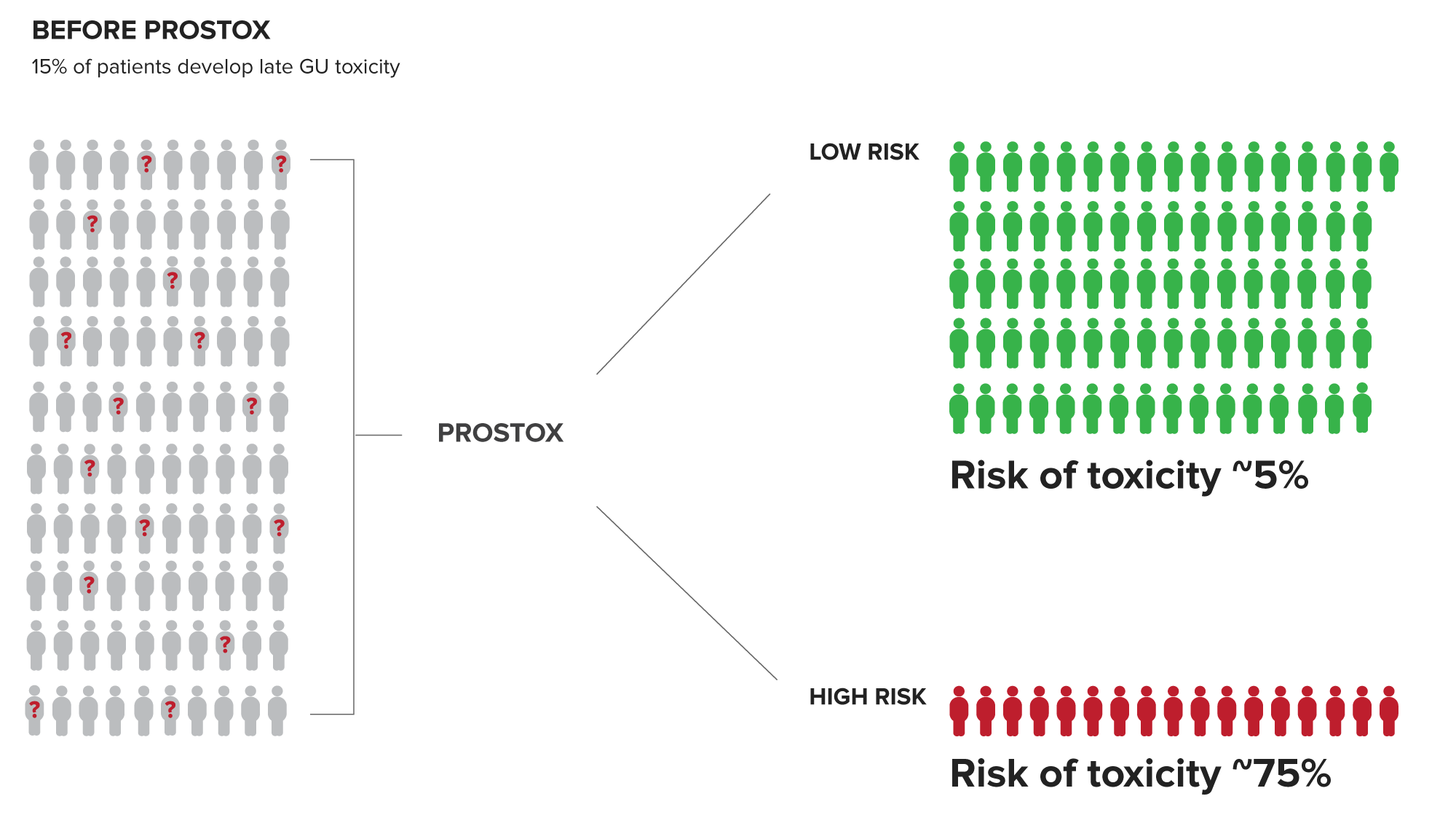
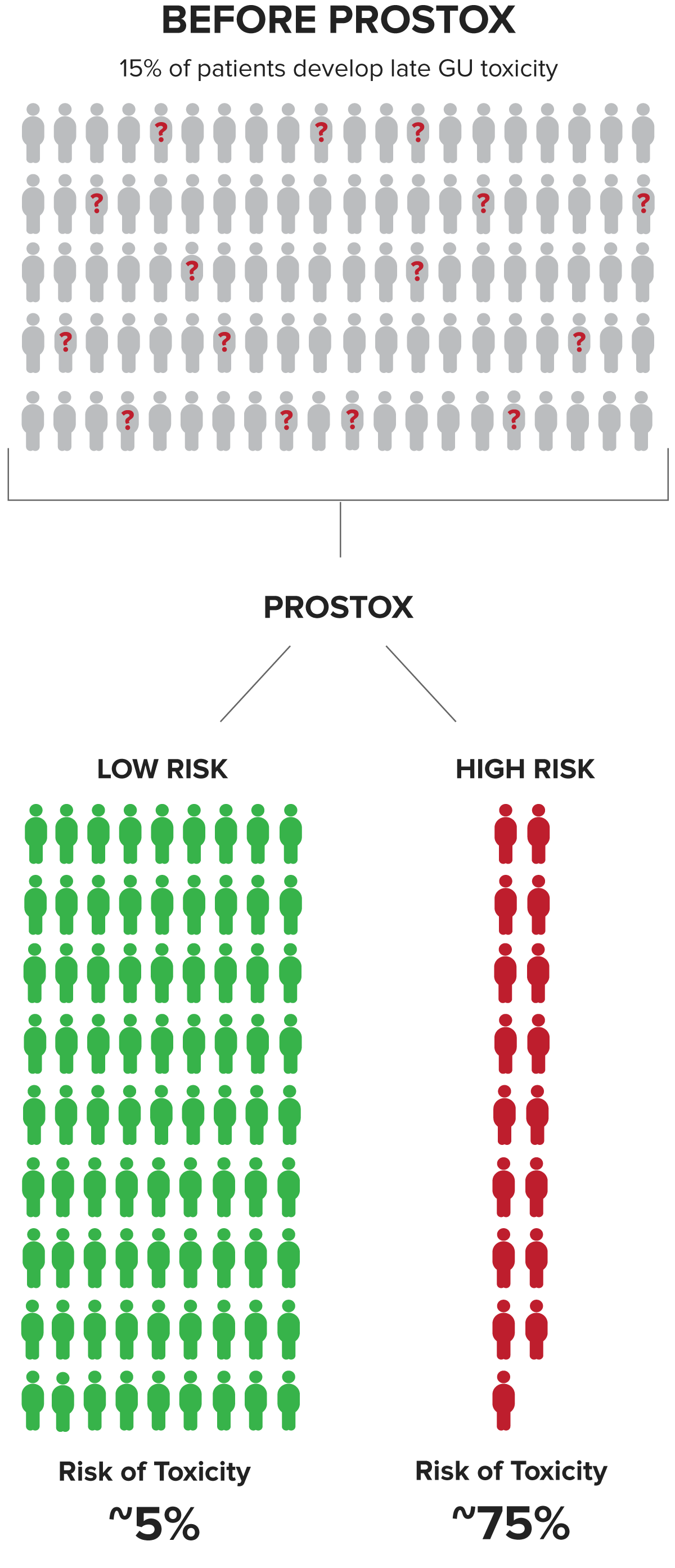
Patients at high risk have a ~75% chance of developing toxicity from SBRT or CFRT/MHFRT. This valuable insight enables the risk of toxicity to be part of the cancer treatment consideration.
Discover Precision, Clarity, and Confidence
Analysis of clinical validation of PROSTOX CFRT performance.
94%
SPECIFICITY*
75%
PPV*
Positive Predictive Value
89%
NPV*
Negative Predictive Value
10Fold
LESS TOXICITY**
Late grade ≥ 2 GU
*Kishan, A.U. et al. International Journal of Radiation Oncology, Biology, Physics, Volume 123, Issue 1, e615.
**Kishan, A. U. et al. Radiother Oncol 2022.
Evidence
Explore the research and learn how PROSTOX can impact your and your patients’ treatment selection.
2025 | Economic Evaluation
Economic Evaluation of a Novel MicroRNA-Based Assay to Determine Risk of Late Genitourinary Radiation Toxicity in Patients With Prostate Cancer
Health economic analysis
PROSTOX Ultra shows significant heatlh system cost savings and quality-of-life gains for prostate cancer patients undergoing radiaion therapy.
View2025 | PROSTOX Ultra high-risk study
Treatment Choices and Toxicity Outcomes in Patients with a High Risk PROSTOX Score
Prospective cohort study
Patients identified as genetically at high-risk for toxicity after SBRT do not appear to be at higher risk of toxicity after MHFRT or CFRT.
View2025 | Clinical Validation
PROSTOX, a signature of late GU toxicity after SBRT radiotherapy in MIRAGE, a prospective trial
Phase III, randomized clinical study
PROSTOX can accurately predict late Grade ≥ 2 GU toxicity after SBRT, regardless of treatment modality.
View2023 | Clinical Utility
The impact of a genetic signature of late radiation toxicity on prostate cancer treatment decision making
Phase II, single-center, prospective clinical study
PROSTOX predicts toxicity risk from SBRT or CFRT and influences treatment decisions for localized prostate cancer.
View2023 | POST-OP Clinical Validation
Application of a genetic signature of late GU toxicity in SCIMITAR, a Post-op SBRT trial
Phase II, dual-center, single-arm clinical study
PROSTOX identifies a higher risk for late grade ≥ 2 genitourinary (GU) toxicity following post-prostatectomy SBRT.
View2022 | Development Study
Germline variants disrupting microRNAs predict long-term GU toxicity after prostate cancer radiation
Developmental Study
Germline mirSNP-based predictive models effectively forecast late-grade ≥ 2 GU toxicity following CFRT or SBRT.
ViewPROSTOX
