Determine Safest Course of Radiation Treatment for Your Prostate Cancer Patients
Most men with prostate cancer live long after diagnosis. In fact, 95% of them are thriving ten years after being diagnosed. While radiotherapy is an effective treatment, more than 15% of men may experience side effects three months or later after therapy, which can negatively impact their quality of life. Choosing the right treatment option is important to ensure a future where the highest standard of living is not only a possibility but the likely outcome.
Personalized Prostate Cancer Radiation Treatment Decision Workflow
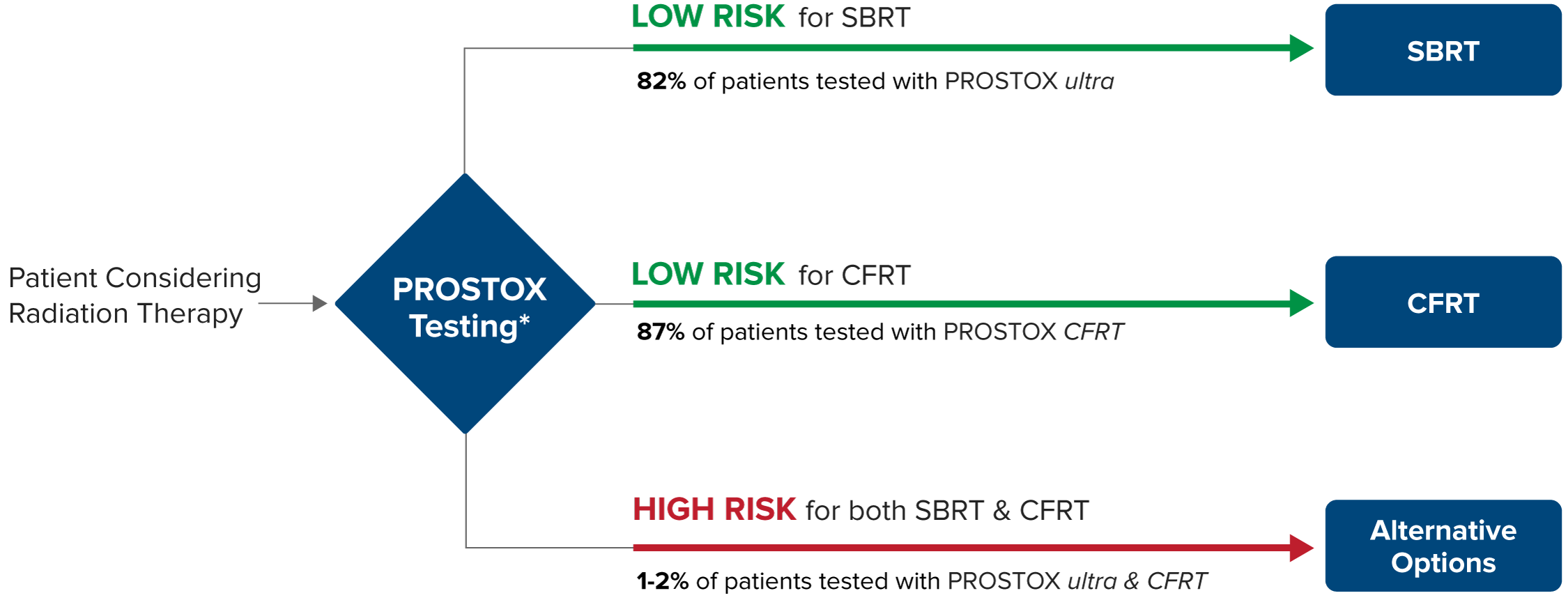
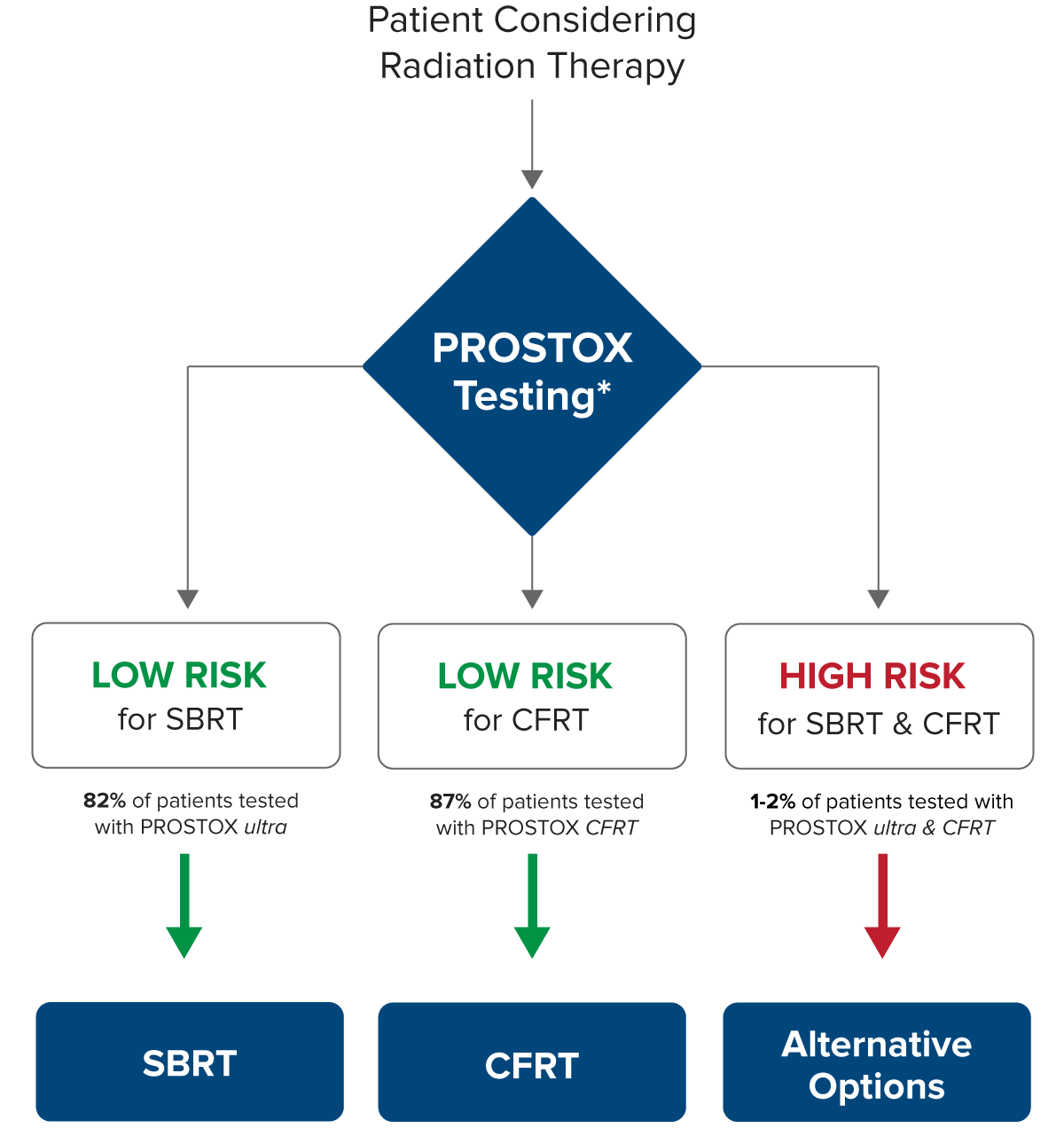
Although efficacy rates are similar between SBRT and CFRT, an individual’s susceptibility to side effects can differ for these treatments. Using unique genetic biomarker panels, PROSTOX ultra and PROSTOX CFRT can identify individuals at risk of toxicity for each treatment type. Recognizing when a treatment may be unsafe helps in selecting a safer alternative. Furthermore, in the rare case that both SBRT and CFRT are unsuitable, other treatment options are available.
Prioritizing Patient Care
Empowering physicians to reduce radiation toxicity risk and enhance long-term patient health.
2,800+
PATIENTS TESTED
20+
MEDICAL CENTERS
Offer PROSTOX Testing
10Fold
LESS TOXICITY
Late grade ≥ 2 GU
When we initially discovered PROSTOX, the data were exciting, but a frequent question that rose is whether a test designed to predict toxicity following radiation delivered years ago would be applicable even in the context of ultra-modern, high precision radiotherapy. Of course, if PROSTOX were truly a test of the biological response to radiation — which we believed was the case — then PROSTOX would be predictive in men treated on a contemporary trial. The validation of PROSTOX as a predictive biomarker in the MIRAGE trial cohort, which ran from 2020-2021, tells us that a genetic proclivity to increased toxicity after radiation persists even with modern, advanced SBRT, including MRI-guided SBRT. This substantiates that it is a true test of biology.”
Dr. Amar Kishan, MD, Radiation Oncologist
Executive Vice Chair for the Department of Radiation Oncology at the David Geffen School of Medicine at UCLA and the UCLA Jonsson Comprehensive Cancer Center

PROSTOX
